Christine D. Lukac, David Twa
ABSTRACT
In Canada, lymphomas are the fifth most prevalent cancer and the incidence of this heterogeneous group of malignancies is increasing. Though recent advances in allopathic medicine with molecularly precise therapies have improved patient survival, many lymphoma patients still succumb to their disease. Often patients also experience reduced quality of life as a result of their cancer and allopathic treatment–related side effects. In light of these outcomes, studies have reported that patients frequently use complementary and alternative medicine (CAM) to help manage their disease. As most patients elect to engage in CAM concurrently with allopathic therapy, it is necessary to consider common CAM modalities that may have outright and/or synergistically harmful side effects that limit the efficacy of allopathic therapy in treating lymphomas. Despite limited scientific evidence supporting CAM efficacy, healthcare providers should still acknowledge the reasons for why patients might choose to use CAM. Here, we examine recent findings on prevalence, rationale, and contraindications for CAM usage by lymphoma patients. Taken together, we believe this analysis may facilitate informed discussion on the disadvantages and advantages of CAM, and when it might be used to appropriately manage lymphomas and allopathic treatment–related symptoms.
INTRODUCTION
Lymphoma is a type of adaptive immune cell cancer that, unlike leukemia, usually manifests as a fleshy tumour in lymphoid organs.[1,2] The majority of lymphomas are derived from the B–cell lineage and can be categorized as either Hodgkin or non–Hodgkin lymphomas.[1,2] In Canada, lymphomas rank fifth in prevalence with over 9,000 incident diagnoses in 2014 alone.[3] As with other solid cancers, therapeutic mainstays include radiation, cytotoxic, and immune modulatory therapeutics, and stem cell transplantation.[2] Of note are the latest advances in molecularly precise biological medicine (e.g. Rituxan®/rituximab) that specifically target malignant cells while sparing surrounding tissue. Although instrumental in improving overall patient survival, such allopathic therapies (alternatively and perhaps inappropriately termed conventional, Western or mainstream therapies) are incapable of successfully managing all lymphoma patients.[4] Indeed, treatment–related side effects that are both immediate and long–term (e.g. secondary cancers, organ damage/failure, and opportunistic infections) are of ongoing concern.[2] As a result of disease symptoms and allopathic treatment–related side effects, lymphoma patients often choose complementary and alternative medicine (CAM) to derive a perceived benefit in lymphoma management.[5]
Here, we define CAM as encompassing non–allopathic supplements (including vitamins/minerals and herbs) and manual manipulative practices (acupuncture, massage and traditional medicine, among others). The medical community recognizes two methods of implementing CAM: (1) complementary therapy which involves the concurrent use of allopathic therapy and (2) alternative therapy which sees the complete replacement of allopathic therapy.[6] While the vast majority of cancer patients fall into the former category, the lack of peer–reviewed, randomized control clinical trials assessing the efficacy of CAM against gold standards of care underlies the concerns of healthcare practitioners.[7‑11] Especially concerning are the inherent toxicities of certain complementary therapies, their harmful drug synergies with allopathic therapies, and the resulting depressed efficacy of allopathic anti–cancer treatments.[6,12] Furthermore, while studies cite the placebo effect and patient empowerment as potential benefits of CAM usage, these advantages must be ethically weighed against the potential harms of propagating misinformation and false hope.[13] Finally, some CAM modalities remain unregulated in cost, administration, and adherence to safety standards. This may ultimately expose patients to risky and unsound healthcare practices in CAM delivery, as has been reported previously.[14]
In spite of these and other concerns, CAM should still not be dismissed without critical appraisal; some patients appear to derive benefit from CAM and too little is known of CAM to rule out its place in healthcare.[5,11,15,16] Between physicians and their patients, CAM therapies should be discussed and assessed scrupulously on a case–by–case basis to determine the best comprehensive treatment plan for the management of lymphoma. To help inform such discussions and decisions, we examine three questions regarding CAM in lymphoma treatment: (1) what forms of CAM are most frequently used, (2) what are the intentions of patients when engaging in CAM, and (3) what potential contraindications exist for CAM therapies.
- Prevalence
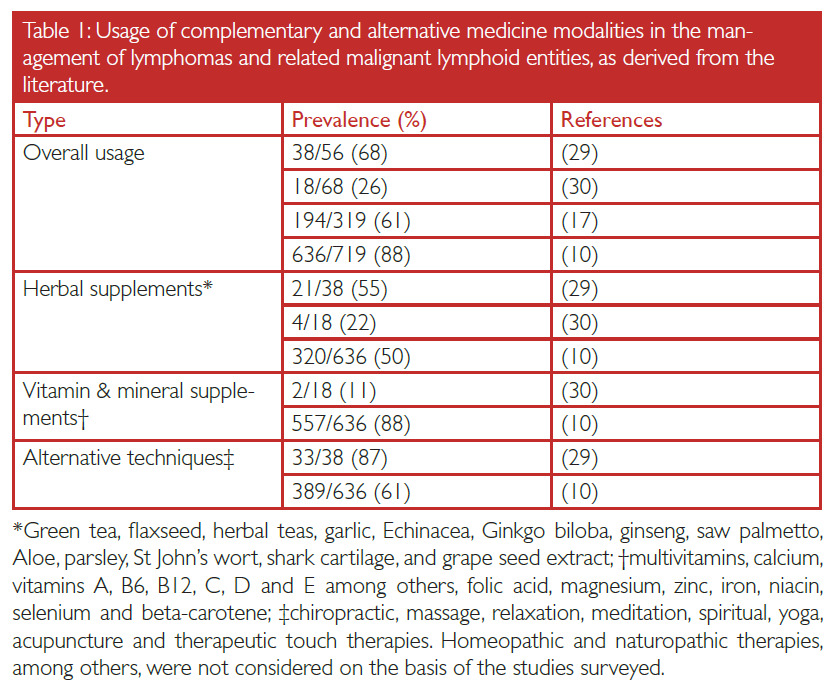
Large–scale studies surveying patients with several types of cancer have reported a prevalence of CAM usage as high as 80 %, with the majority of such patients engaging in the complementary form of therapy administration.[5,11] Among lymphoma patients, a range of 26‑88 % has been reported across four peer–reviewed studies, each surveying at least 50 lymphoma or lymphoma–related entities (Table I). In 1,162 cumulative cases across these studies, 76 % of patients reported using some form of CAM therapy (Table I). Two studies assessing distribution of usage by sex found significantly greater usage of CAM among women than men (P < 0.0001 and P = 0.009).[10,17] No surveyed study reported any significant correlation between CAM usage and lymphoma stage, although one paper found increased CAM usage associated with T– and natural killer cell–derived lymphomas (P = 0.04), both of which are known to have a more aggressive clinical course.[2,10]
In assessing three major modalities of CAM therapy, vitamins/minerals (e.g. β–carotene and selenium) were most frequently employed among 85 % of patients across the surveyed studies. This was followed by manual manipulative CAM techniques (e.g. acupuncture and massage) at 62 % and herbal supplements (e.g. garlic and Ginkgo biloba) at 50 % (Table I). While most of the information derived from the distribution CAM usage among lymphoma patients is based on the study by Rausch Osian et al., which had the greatest sample size (N = 719), the prevalence of CAM usage is important in considering any harmful drug interactions with allopathic therapies, as discussed below.[10]
- Rationale
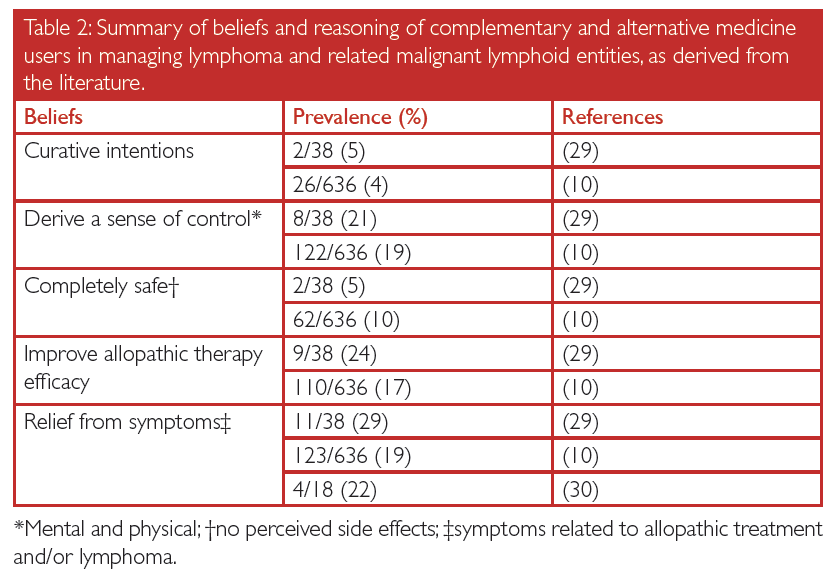
Based on the studies surveyed, the intentions of lymphoma patients engaging in CAM were varied. They included: a perceived reduction in side effects resulting from allopathic therapy and from the cancer itself, improving the efficacy of allopathic treatments, deriving a sense of control in managing their disease, and engaging in CAM with curative intentions (Table II). To date, the authors are not aware of any peer–reviewed, prospective randomized control trials that have found significant association between any of the aforementioned patient intentions and any modality of CAM for the management of lymphoma. Further, studies have found that as many as 10 % of patients believe that CAM is not associated with any toxic side effects (Table II), in spite of literature suggesting multiple harmful activities of supplemental CAM modalities resulting either directly from their use or in synergy with allopathic drugs.
- Select, known contraindications
As has been discussed, a substantial proportion of lymphoma patients engage in CAM concurrently with allopathic treatments; many do so based on scientifically unsupported claims and do not acknowledge the potentially toxic and hazardous side effects (Table II). Compared to some perceived CAM activities that are anecdotally believed to improve patient health however, several means by which CAM therapies can negatively alter clinical course have been studied and have been scientifically reproduced.
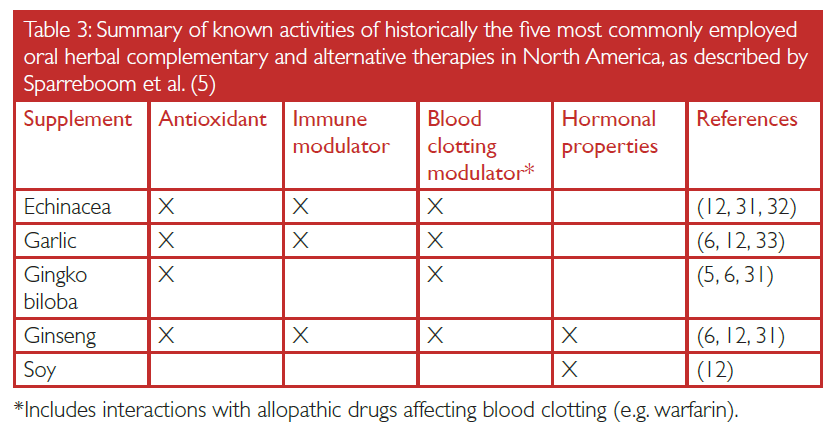
Molecularly precise therapies aside, two established allopathic lymphoma modalities are gamma radiation and anthracycline chemotherapeutics (e.g. Adriamycin®/doxorubicin).[2] One way these therapeutics induce neoplastic cell death is through the generation of reactive oxygen species (ROS).[6,18,19] In cellular nuclei, ROS interact with chromatin, resulting in a level and quantity of DNA damage that surpasses the threshold of DNA repair mechanisms and triggers the apoptotic cascade.[20] Unwanted treatment–related side effects result from both the subsequent release of cancerous apoptotic cell debris and the unintentional destruction of healthy cells as a result of the imprecise nature of radiation and non–targeted chemotherapy.[2] Although conventional therapies can help manage some side effects, lymphoma patients occasionally employ CAM supplements with antioxidant properties, in spite of limited evidence supporting their efficacy.[6] Beyond limited efficacy, some evidence exists suggesting that CAM therapies with antioxidant properties are associated with worse patient outcomes.[6] Studies in head and neck cancers have shown the concurrent use of allopathic therapy with the antioxidants β–carotene and/or vitamin E was associated with more frequent cancer relapse.[21‑25] Although the mechanism is incompletely understood, antioxidant CAM therapies presumably counteract the activity of therapeutically–generated ROS, lessening efficacy. The means by which CAM and allopathic therapies are synergistically absorbed, metabolized, and excreted (termed pharmacokinetics), adds to the complexity in understanding these drug interactions and is an important area of ongoing research. Lessons learned from future pharmacokinetic studies can be applied paradigmatically to instances where antioxidant CAM therapies are taken concurrently with ROS–generating allopathic modalities, as is the case in lymphoma management. The most commonly employed herbal CAM supplements with antioxidant properties are listed in Table III for reference.
CAM supplemental therapies are also known to possess blood clot modulating and hormone signaling properties, in addition to immune modulating activities (Table III).[5,6,12] CAM therapies recognized as immune system modulators are of particular relevance to lymphomas. This is not only because the neoplasm is derived from immune cells but also because the surrounding tumour microenvironment is composed of a heterogeneous reactive cellular infiltrate susceptible to immune modulation. The complexity of the tumour microenvironment is not to be underestimated; paradoxically, certain tumour microenvironment gene signatures have been variably associated with both favorable and unfavorable prognoses.[1,2,26] As such, long–term usage of CAM therapies with immune modulatory properties can produce results opposite of those historically ascribed by CAM practitioners. Prolonged use of Echinacea, for example, has been shown to reduce the number of circulating white blood cells.[27] Furthermore, some immune modulatory CAM compounds can reduce the efficacy of lymphoma restaging, as is the case with the concurrent use of Nerium oleander and positron emission tomography.[28] Taken together, caution should be exercised as there is little scientific data demonstrating how immune modulating CAM therapies might reshape the composition of the tumour microenvironment.
Contraindicated CAM usage is not limited to the oral supplement forms of therapy; CAM techniques, such as acupuncture, can also put patients at risk of unfavorable outcomes. Characteristically, lymphoma patients have a depressed immune system resulting from a combination of allopathic treatment and the population of dysfunctional immune cells that constitute the lymphoma. The few functional immune cells that remain are often insufficient in number and inactive in biological function to respond to infectious agents potentially introduced into the body through acupuncture. Additionally, as noted in the Canadian Cancer Society patient handbook for complementary therapies, massage therapy may be contraindicated for late stage lymphoma and multiple myeloma patients who have cancer infiltrations and lesions that weaken the bone architecture.[2]
CONCLUSION
Based on the literature, it has been observed that as many as 88 % of lymphoma patients engage in some form of CAM therapy, the most common being vitamin/mineral supplements. Most patients employing CAM do so concurrently with allopathic treatments, although the rationale for employing CAM therapy is highly varied. Moreover, up to 10 % of patients do not recognize that CAM therapies may have side effects that could impact their clinical course. In particular, CAM therapies with antioxidant and immune modulatory properties have the potential to negatively interfere with allopathic treatments. As little is understood of the activities and potential contraindications of CAM therapies in the context of lymphoid cancer, these treatments should be assessed on an informed and unbiased case–by–case basis. In so doing, the primary focus can remain on the well–being of the patient with the concurrent development of a safe, agreeable, and efficacious lymphoma management plan that is established on sound scientific principles and clinical practice.
REFERENCES
- Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14(8):517‑34.
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4 ed. Lyon, France: IARC Press; 2008. p. 1‑439.
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2014. Toronto, ON: Canadian Cancer Society; 2014. p. 1‑132.
- Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027‑33.
- Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: potential adverse interactions with anticancer agents. J Clin Oncol. 2004;22(12):2489‑503.
- Michaud LB, Karpinski JP, Jones KL, Espirito J. Dietary supplements in patients with cancer: risks and key concepts, part 1. Am J Health Syst Pharm. 2007;64(4):369‑81.
- Boon H, Stewart M, Kennard MA, Gray R, Sawka C, Brown JB, et al. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. J Clin Oncol. 2000;18(13):2515‑21.
- Carboon I. Rethinking the evidence imperative: why patients choose complementary and alternative medicine. Leuk Lymphoma. 2008;49(2):181‑2.
- Jackson JL, Srinivasan M, Rea J, Fletcher KE, Kravitz RL. The validity of peer review in a general medicine journal. PLoS One. 2011;6(7):e22475.
- Rausch Osian S, Leal AD, Allmer C, Maurer MJ, Nowakowski G, Inwards DJ, et al. Widespread use of complementary and alternative medicine among non-Hodgkin lymphoma survivors. Leuk Lymphoma. 2015;56(2):434‑9.
- Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18(13):2505‑14.
- Michaud LB, Karpinski JP, Jones KL, Espirito J. Dietary supplements in patients with cancer: risks and key concepts, part 2. Am J Health Syst Pharm. 2007;64(5):467‑80.
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686‑95.
- Public safety advisory for clients of acupuncture and Chinese medicine centre in Abbotsford. Fraser Health Newsroom [Internet]. 2014 Mar 1 [cited 2015 Jun 10]. Available from: http://news.fraserhealth.ca/News/November-2014/Public-safety-advisory-for-clients-of-acupuncture.aspx
- Berman BM, Singh BB, Lao L, Langenberg P, Li H, Hadhazy V, et al. A randomized trial of acupuncture as an adjunctive therapy in osteoarthritis of the knee. Rheumatology (Oxford). 1999;38(4):346‑54.
- Irving KB. Cancer and quackery. Intern Med J. 2012;42(4):466‑8.
- Hamilton AS, Miller MF, Arora NK, Bellizzi KM, Rowland JH. Predictors of use of complementary and alternative medicine by non-Hodgkin lymphoma survivors and relationship to quality of life. Integr Cancer Ther. 2013;12(3):225‑35.
- Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261(7):3068‑74.
- Mendivil-Perez M, Velez-Pardo C, Jimenez-Del-Rio M. Doxorubicin induces apoptosis in Jurkat cells by mitochondria-dependent and mitochondria-independent mechanisms under normoxic and hypoxic conditions. Anticancer Drugs. 2015;26(6):583‑98.
- Nakano H, Yonekawa H, Shinohara K. Threshold level of p53 required for the induction of apoptosis in X-irradiated MOLT-4 cells. Int J Radiat Oncol Biol Phys. 2007;68(3):883‑91.
- Bairati I, Meyer F, Gélinas M, Fortin A, Nabid A, Brochet F, et al. Randomized trial of antioxidant vitamins to prevent acute adverse effects of radiation therapy in head and neck cancer patients. J Clin Oncol. 2005;23(24):5805‑13.
- Bairati I, Meyer F, Gélinas M, Fortin A, Nabid A, Brochet F, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst. 2005;97(7):481‑8.
- Bairati I, Meyer F, Jobin E, Gélinas M, Fortin A, Nabid A, et al. Antioxidant vitamins supplementation and mortality: a randomized trial in head and neck cancer patients. Int J Cancer. 2006;119(9):2221‑4.
- Ferreira PR, Fleck JF, Diehl A, Barletta D, Braga-Filho A, Barletta A, et al. Protective effect of alpha-tocopherol in head and neck cancer radiation-induced mucositis: a double-blind randomized trial. Head Neck. 2004;26(4):313‑21.
- Meyer F, Bairati I, Fortin A, Gélinas M, Nabid A, Brochet F, et al. Interaction between antioxidant vitamin supplementation and cigarette smoking during radiation therapy in relation to long-term effects on recurrence and mortality: a randomized trial among head and neck cancer patients. Int J Cancer. 2008;122(7):1679‑83.
- Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875‑85.
- Kemp DE, Franco KN. Possible leukopenia associated with long-term use of echinacea. J Am Board Fam Pract. 2002;15(5):417‑9.
- Akkas BE, Kitapci MT, Arpaci F, Gurses MA, Unlu N. Complementary and alternative medical therapies can be potential pitfalls for PET/CT imaging: report of false-positive FDG PET/CT findings caused by Nerium oleander vaccine in a patient with lymphoma. J Altern Complement Med. 2013;19(11):916‑7.
- Habermann TM, Thompson CA, LaPlant BR, Bauer BA, Janney CA, Clark MM, et al. Complementary and alternative medicine use among long-term lymphoma survivors: a pilot study. Am J Hematol. 2009;84(12):795‑8.
- Molassiotis A, Margulies A, Fernandez-Ortega P, Pud D, Panteli V, Bruyns I, et al. Complementary and alternative medicine use in patients with haematological malignancies in Europe. Complement Ther Clin Pract. 2005;11(2):105‑10.
- Masteikova R, Muselik J, Bernatoniene J, Bernatoniene R. Antioxidative activity of Ginkgo, Echinacea, and Ginseng tinctures. Medicina (Kaunas). 2007;43(4):306‑9.
- Abdul MI, Jiang X, Williams KM, Day RO, Roufogalis BD, Liauw WS, et al. Pharmacokinetic and pharmacodynamic interactions of echinacea and policosanol with warfarin in healthy subjects. Br J Clin Pharmacol. 2010;69(5):508‑15.
- Wu ZR, Peng C, Yang L, Li JY, Xin W, Yong W, et al. Two cinnamoyloctopamine antioxidants from garlic skin attenuates oxidative stress and liver pathology in rats with non-alcoholic steatohepatitis. Phytomedicine. 2015;22(1):178‑82.
Lukac CD, David Twa D. Complementary and Alternative Medicine in the Management of Lymphomas: Prevalence, Rationale, and Contraindications. UBCMJ. 2015: 7.1 (52-55).